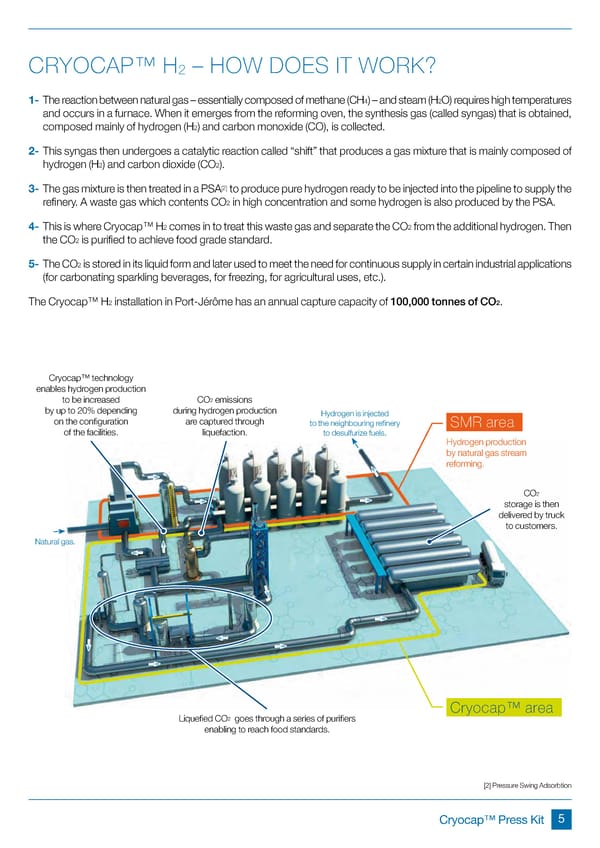
CRYOCAP™ H – HOW DOES IT WORK? 2 4 2 1- The reaction between natural gas – essentially composed of methane (CH ) – and steam (H O) requires high temperatures and occurs in a furnace. When it emerges from the reforming oven, the synthesis gas (called syngas) that is obtained, 2 composed mainly of hydrogen (H ) and carbon monoxide (CO), is collected. 2- This syngas then undergoes a catalytic reaction called “shift” that produces a gas mixture that is mainly composed of 2 2 hydrogen (H ) and carbon dioxide (CO ). [2] 3- The gas mixture is then treated in a PSA to produce pure hydrogen ready to be injected into the pipeline to supply the 2 refinery. A waste gas which contents CO in high concentration and some hydrogen is also produced by the PSA. 2 2 4- This is where Cryocap™ H comes in to treat this waste gas and separate the CO from the additional hydrogen. Then 2 the CO is purified to achieve food grade standard. 2 5- The CO is stored in its liquid form and later used to meet the need for continuous supply in certain industrial applications (for carbonating sparkling beverages, for freezing, for agricultural uses, etc.). 2 2 The Cryocap™ H installation in Port-Jérôme has an annual capture capacity of 100,000 tonnes of CO . Cryocap™ technology enables hydrogen production 2 to be increased CO emissions by up to 20% depending during hydrogen production Hydrogen is injected on the configuration are captured through to the neighbouring refinery SMR area of the facilities. liquefaction. to desulfurize fuels. Hydrogen production by natural gas stream reforming. 2 CO storage is then delivered by truck to customers. Natural gas. Cryocap™ area 2 Liquefied CO goes through a series of purifiers enabling to reach food standards. [2] Pressure Swing Adsorbtion Cryocap™ Press Kit 5
 Cryocap Page 4 Page 6
Cryocap Page 4 Page 6